H2 Deck By Bold Name
h2 xxxxxx
H1 xxxxxx
h2 xxxxx
Test & INspection


When testing the emerging wearable devices, manufacturers are now confronted with various new and unique challenges. By James Ritchey
Evolving Needle-Based Drug Delivery Products & Testing Methods
Medical
H2 Deck Info By Paragraph Style Bold
Headline
Pioneers in the pharmaceutical industry quickly understood the medical benefits of parenteral drug delivery. Decades worth of evolving systems that promised to optimize and fine-tune efficacy, safety, and patient comfort precede today’s on-body-delivery systems. And as the industry continues to move the needle forward to meet even higher expectations while adhering to increasingly intricate and strict standard requirements, collaborative efforts from testing equipment manufacturers remain essential.
From the early invention of glass syringes in the 1940s to the introduction of autoinjector technology for military use in the 1970s—subsequently leading to the creation of the EpiPen—needle-based drug delivery has been evolving to provide easy-to-administer and pain-minimizing results for patients. This evolution has included both an increasing number of patient indications as well as a growing level of patient usage compliance. In fact, autoinjector usage rates have steadily risen at over 20% per year for the last few decades.
As the industry continues to trend toward a more patient-centric approach, we see an increasing buzz around the development and utilization of wearable injectors, also known as on-body delivery systems (OBDS)—the next evolution in needle-based drug delivery products. While wearable pump solutions for subcutaneous drug delivery are already being widely used in the insulin/diabetes market, they are now expanding into oncology, treatment for autoimmune diseases and neurological disorders, and other disease state areas.

The evolution from autoinjector to OBDS requires new testing solutions for buzzer sounds, LEDs, motor drives, expanded injection times (seconds to minutes), adhesion, and more.
Key drivers in the increasing usage of the OBDS include the improvement in patient compliance to treatment, the growing movement toward home care—providing patients more flexibility in terms of drug administration from home versus repeated visits to a clinic—and the accompanying reduction in the cost of therapy. This movement is further supported by the development of new drugs, such as biologics which tend to have a higher viscosity and are often delivered over an extended period of time.
As the pharmaceutical market evolved, from the first syringe to current OBDS systems, intelligent testing solutions from leading manufacturers have developed alongside them and supported continuous improvements in quality with efficient systems and unique, proven test concepts. Although the OBDS are relatively new in concept within the scope of parenteral drug delivery, the confidence that they are truly the next wave in devices is supported by the already established ISO testing standards and unique testing systems that are available to evaluate them.
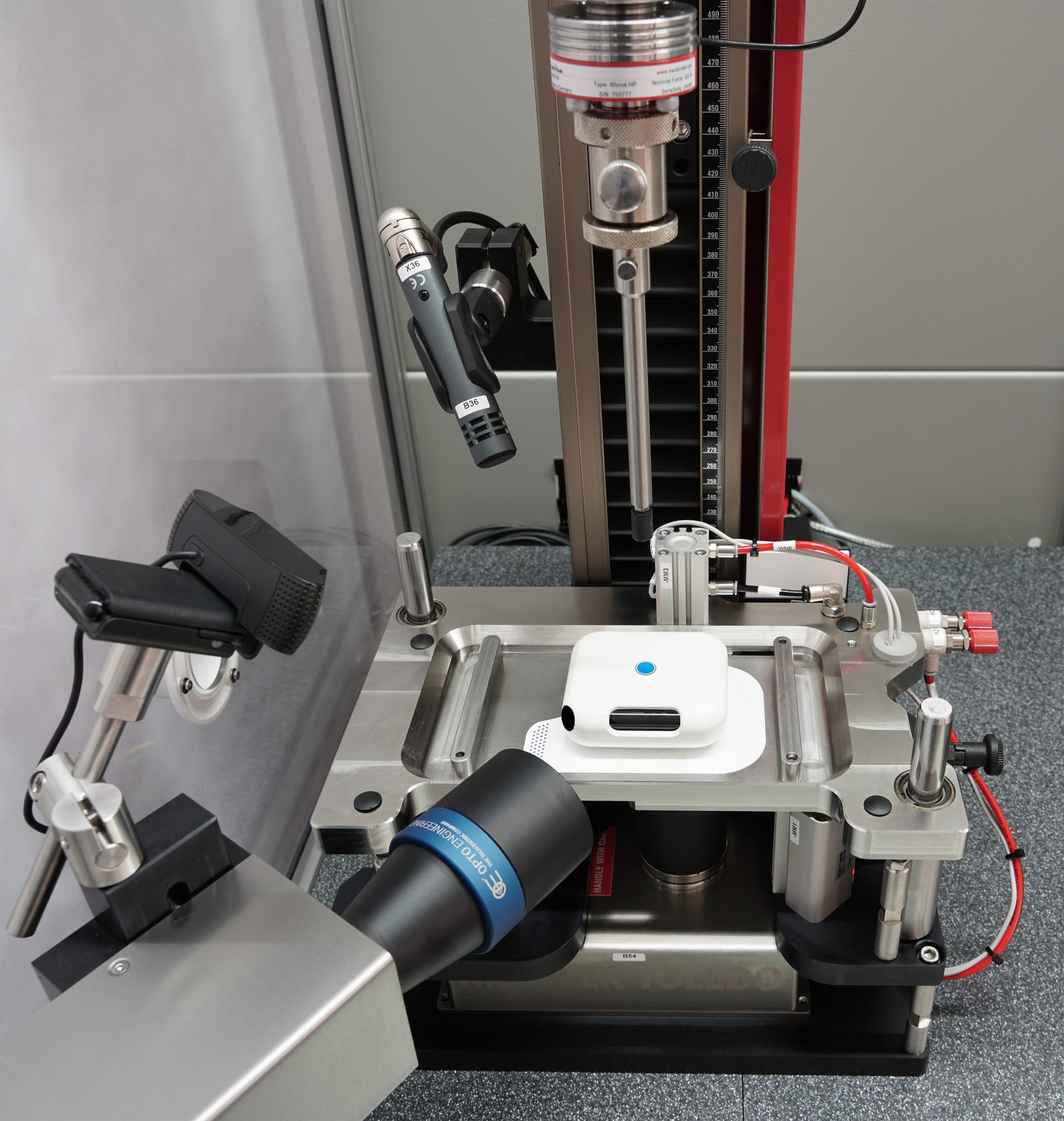
ZwickRoell zwickiLine testing machine for OBDS testing technology with high-resolution measurement devices, cameras, and microphones that must be verified on a daily basis.
All needle-based delivery systems are designed to meet global industry standards to ensure safety, functionality, and overall quality of the device. Injectables have traditionally been covered by ISO 11040 for prefilled syringes and the ISO 11608 series for insulin pens, pen injectors, and autoinjectors. ISO 11040 is divided into various sections, each of which is further specialized, i.e. section four for glass barrel syringes, section six for plastic barrel devices and section eight for syringes ready to be used. Within those sections, individual annexes lay out the test details for breakaway and glide forces, needle penetration and pullout forces, and flange and Luer cone breakage resistance to name a few. ISO 11608 also contains several annexes to cover pens and autoinjectors, and presents test details including dose accuracy, injection times, needle length and more. With the emergence of OBDS drug delivery systems, the ISO 11608 committee expanded the standard to include a sixth annex (11608-6) to cover these types of combination products.
Specifically, the latest (2022) version of the ISO 11608 standard eliminates reference to the term “essential performance” and instead refers to “primary functions”, which it defines as “those functions for which failure would directly result in new and unacceptable harm.” With this change, the standard focuses on unacceptable harm, and not just risk. One of the essential primary functions of an on-body delivery system, which is explicitly emphasized in the standard, is dose accuracy. ISO 11608-6 addresses three OBDS characteristics that are relevant to dosing: dose accuracy, dose delivery time and dose delivery profile. Testing the dose delivery profile—defined in the standard as the relationship between the volume delivered and time throughout the delivery—requires a special test equipment configuration.
Not only does this primary function have to be tested in accordance with FDA guidelines, but essential performance requirements must also be included in the design verification process. This means that the delivery device must be subjected to a variety of tests under consideration of preconditions and ageing effects, to arrive at true device function results.

ZwickRoell OBDS testing setup
When testing the emerging wearable devices, manufacturers are now confronted with various new and unique challenges. ISO 11608-6 requires manufacturers to measure the amount of the drug dispensed, which is directly influenced by the overall function of the various mechanical components of an OBDS. These devices typically hold much higher volumes of medication, which translates into longer injection times, sometimes lasting as long as an hour. Measuring the medication flow rate will be a critical component of the combination products’ capabilities as high flow rates can be painful for the patient. Additionally, the needles within these OBDS devices tend to be both larger in diameter and shorter in length which will require higher resolution measurement techniques. Lastly, these devices will be adhered to the body for extended periods of time so testing of the adhesive properties at body temperature will be important to track.
The patient-treatment promise and benefits surrounding these emerging drug delivery systems charged with innovative features and functionality drive manufacturers to overcome the newly presented testing challenges they now face. For a high-level perspective, we can divide these challenges into three primary categories:
- Detailed and proper interpretation of the 2022 edition of the ISO 11608, in particular annex 6 and deriving the respective primary functions of the device to be tested. Many of these functions were described above.
- Establish the technical features for the testing machines that support the new requirements. This includes consideration for essential performance requirements such as testing sensors, LEDs and sounds on the device.
- Proper evaluation of the acquired data. In the past, many companies or delivery device manufacturers used test stations for individual components of the device. One of the key challenges stemming from this approach is coordinating data and results from different individual tests.
Companies need sustainable strategies for data collection and analysis and finding a consolidated, single-system testing scheme may turn out to be essential.

ZwickRoell semi-automated testing solution including custom device holding fixtures, precision control electronics, and multi-purpose testing software including ISO 11608-6 methods
A solution has to work for not only the manufacturer but also partners in terms of reproducibility among all parties involved. Furthermore, by automating testing processes, human influences on the test result are eliminated to the highest extent possible and higher accuracy is ensured. The use of intelligent software integrated in the test sequence documents results and clearly displays them for the user. For maximum transparency, reliable traceability for validation and quality control, machines must guarantee reliable performance in accordance with standards like 21 CFR Part 11. This is particularly important and essential in the biotechnology and pharmaceutical industries to trace who did what, when and why, and who is responsible. Through close collaboration between delivery device manufacturers and testing machine providers and by continuously sharing expertise and know-how, the industry will continue to evolve medicine and needle-based drug delivery devices and ultimately benefit patients to the greatest extent possible.
